[ad_1]
Mammogram facilities will need to follow some new rules soon.
The U.S. Food and Drug Administration (FDA) on Thursday released updated regulations that require mammogram providers to notify patients about the density of their breast tissue.
The updates apparently will give the FDA more oversight as well over individual mammography facilities — with the purported goal of improving patient care and communications.
During a screening mammogram, a technician uses a special X-ray machine to detect any abnormalities that could indicate a risk of breast cancer. The images show whether a woman has dense breast tissue, which can make it more difficult to spot cancer warning signs.
BREAST CANCER AND MAMMOGRAMS: EVERYTHING YOU NEED TO KNOW ABOUT THE DISEASE, SCREENING AND MORE
Women with dense breast tissue are also at a higher risk of developing the disease.
“Today’s action represents the agency’s broader commitment to support innovation to prevent, detect and treat cancer,” said Hilary Marston, M.D., M.P.H., chief medical officer of the FDA in Silver Spring, Maryland, in a press release announcing the new regulations.
“Since 1992, the FDA has worked to ensure patients have access to quality mammography.”
The U.S. Preventive Services Task Force recommends that women 50 years or older should get mammograms every other year. For those with a family history of the disease, the recommendation is to begin at age 40. (iStock)
“The impact of the Mammography Quality Standards Act on public health has been significant, including a steep decrease in the number of facilities that do not meet quality standards,” she continued.
Mammography facilities will have 18 months to comply with the new regulations.
“This means that more women have access to consistent, quality mammography. We remain committed to advancing efforts to improve the health of women and strengthen the fight against breast cancer.”
MISSOURI BILL TO EASE ACCESS FOR BREAST CANCER SCREENINGS
Anne Peled, M.D., a breast cancer surgeon and co-director of the Breast Care Center of Excellence at Sutter Health California Pacific Medical Center in San Francisco, said she recognizes the importance of the FDA’s new guidance.
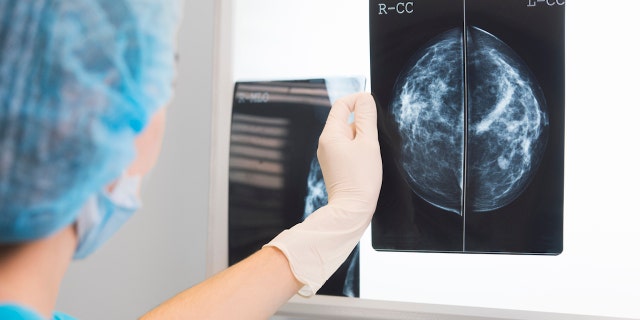
Roughly half of U.S. women over age 40 have dense breast tissue, which can make it more difficult for technicians and others to spot breast cancer warning signs. (iStock)
“As a patient, knowing you have denser breast tissue allows you to talk to your health care team about whether additional breast cancer screening would be helpful and come up with a plan tailored for you,” she told Online News 72h Digital in an email.
“Having oversight of consistency in reporting on density and making sure people with denser breasts have access to technology like 3D mammograms is essential for improving equity in breast cancer screening.”
Roughly half of U.S. women over age 40 have dense breast tissue, the FDA noted.
CANCER BLOOD TEST USING DNA FRAGMENTS BRINGS HOPE FOR EARLIER DETECTION, SAY RESEARCHERS
Jennifer Hartman, an Indiana-based nurse practitioner specializing in surgical breast oncology, said she believes requiring notification of dense breast tissue is a step in the right direction.
She said she also believes it’s important to provide women with affordable access to additional screening — such as breast ultrasounds or MRIs — if they do have dense breast tissue.
Since 1990, mammograms have helped reduce breast cancer deaths by nearly 40%.
“While understanding and being educated on breast density provides value, it will be even more life-saving if we could provide additional actionable measures based on that knowledge,” she told Online News 72h Digital in an email.

The new updates will give the FDA greater oversight over the facilities offering mammograms, with a focus on enforcing regulations and ensuring proper communication with patients. (iStock)
Regarding the FDA updates, mammography facilities will have 18 months to comply with the new regulations.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
Each year in the U.S., about 264,000 cases of breast cancer are diagnosed in women and around 42,000 women die of the disease, according to the CDC.
The U.S. Preventive Services Task Force recommends that women 50 years or older should get mammograms every other year.
For those with a family history of the disease, the recommendation is to begin mammograms at 40 years old.
CLICK HERE TO GET THE Online News 72h APP
Since 1990, mammogram screenings have helped to reduce breast cancer deaths by nearly 40%, according to the American College of Radiology.
[ad_2]
Source link
