[ad_1]
The U.S. Food and Drug Administration (FDA) has authorized a single booster dose of the Pfizer-BioNTech COVID-19 vaccine bivalent for children six months old through four years of age.
At least two months prior to getting this booster, children must have completed their three-dose primary vaccination series.
Children in that age range who only received the first two doses of the Pfizer COVID-19 vaccine are not currently eligible for this booster. The FDA recommends that they complete the three-dose series.
SLEEP DEPRIVATION COULD REDUCE VACCINE ANTIBODIES, NEW STUDY FOUND
The FDA said in its announcement that the Pfizer-BioNTech COVID-19 Vaccine, Bivalent includes an mRNA component that provides a “broadly protective” immune response against COVID-19, as well as against the omicron variants BA.4 and BA.5.
The Food and Drug Administration (FDA) has authorized a single booster dose of the Pfizer-BioNTech COVID-19 bivalent vaccine for children six months through four years of age who have previously completed a three-dose primary vaccination series. (iStock)
“Today’s authorization provides parents and caregivers of children 6 months through 4 years of age who received the three-dose primary series with the monovalent Pfizer-BioNTech COVID-19 vaccine an opportunity to update their children’s protection by receiving a booster dose with the Pfizer-BioNTech COVID-19 Vaccine, Bivalent,” said Peter Marks, M.D., PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a press release announcing the authorization.
“Currently available data show that vaccination remains the best defense against severe disease, hospitalization and death caused by COVID-19 across all age groups,” he added.
“We encourage all eligible individuals to make sure that their vaccinations are up-to-date with a bivalent COVID-19 vaccine,” he also said.
COVID-19 SHOCKER: PARENTS LIED ABOUT THEIR KIDS’ SICKNESS STATUS AND BROKE QUARANTINE RULES, STUDY FINDS
Before authorizing the booster, the FDA analyzed immune response data for 60 children in this age range who had completed the three-dose vaccine series in addition to a booster dose.
The FDA also cited a clinical study that evaluated children six months through 11 years of age who completed the three-dose primary vaccine series along with the booster dose of Pfizer-BioNTech COVID 19 Vaccine, Bivalent.

Children must have completed their three-dose primary vaccination series at least two months prior to getting the new booster. (iStock)
The most common side effect among children between six months and 23 months were drowsiness, irritability, pain and swelling, injection site redness, decreased appetite, fatigue and fever, the FDA noted.
For children between two years and four years old, the most common side effects were vomiting, headache, fatigue, injection site pain, diarrhea, redness and swelling, joint pain and chills.
CDC ADVISORY GROUP FINDS INSUFFICIENT EVIDENCE TO RECOMMEND MORE THAN ONE COVID-19 BOOSTER A YEAR
For those ages five through 11, the most common side effects were muscle pain, joint pain, fatigue, headache, fever, chills, diarrhea, vomiting, injection site pain, swelling and redness, and swelling of the lymph nodes.
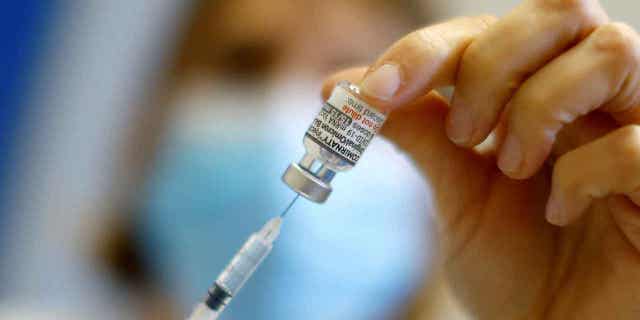
Before authorizing the new booster, the FDA analyzed immune response data for 60 children in this age range who had completed the three-dose vaccine series in addition to a booster dose. (REUTERS/Eric Gaillard)
The FDA also cited several other studies that support the safety of the recommended booster dose.
These studies evaluated individuals older than age 55 who received the booster, children six months and older who received the primary vaccine series, and people five years and older who received booster vaccination with the monovalent Pfizer-BioNTech COVID-19 vaccine, which was previously authorized but is no longer.
“Just because a vaccine is approved doesn’t mean it should automatically be used.”
Marc Siegel, M.D., professor of medicine and medical director of Doctor Radio at NYU Langone Medical Center, as well as a Online News 72h medical contributor, pointed out that just because a vaccine is approved doesn’t mean it should automatically be used.
“That should be a discussion between the parent and pediatrician — and child,” he told Online News 72h Digital in an email.
“It should be based on factors such as the child’s previous reaction to the vaccine.”

For children between two years and four years of age, the most common side effects from the vaccine were vomiting, headache, fatigue, injection site pain, diarrhea, redness and swelling, joint pain and chills. (iStock)
He added that the effectiveness of the vaccines wanes over time.
Fact sheets provided to vaccine recipients, caregivers and health care providers warn of the risk of myocarditis and pericarditis, the FDA noted.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
In February, the Centers for Disease Control and Prevention (CDC) updated its official child and adolescent immunization schedule to include COVID-19 vaccines.
CLICK HERE TO GET THE Online News 72h APP
The schedule, which is posted on the CDC’s website, recommends that children between six months of age and 18 years old should receive two doses of the primary series between four and eight weeks apart — followed by a booster dose at least eight weeks later.
[ad_2]
Source link
